Paricalcitol, ergocalciferol analog
From Wikipedia, the free encyclopedia
- patented in 1989, approved for medical use in 1998
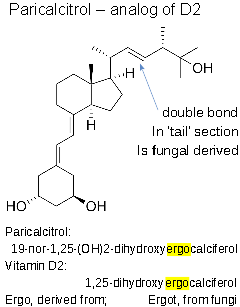
- chemically: 19-nor-1,25-(OH)2-vitamin D2.
- Marketed by Abbott Laboratories under the trade name Zemplar
- drug used for the prevention and treatment of secondary hyperparathyroidism (excessive secretion of parathyroid hormone) associated with chronic kidney failure.
- analog of 1,25-dihydroxyergocalciferol, the active form of vitamin D2 (ergocalciferol).
Medical uses
Its primary use in medicine is in the treatment of secondary hyperparathyroidism associated with chronic kidney disease.
However current evidence is not sufficient to demonstrate an advantage of paricalcitol over non-selective vitamin D derivatives for this indication.
Adverse effects (similar to vitamin D)
Very common (>10% frequency):
- Nausea
Common (1-10% frequency):
Diarrhoea
Edema
Allergic reaction
Arthritis
Dizziness†
Stomach discomfort‡
Gastroesophageal reflux disease†
Acne†
Hypercalcaemia†
Hypocalcaemia†
Hyperphosphataemia
Decreased appetite†
Headache
Breast tenderness†
Taste changes
Hypoparathyroidism
Vertigo
Rash‡
Uncommon (0.1-1% frequency):
Abnormal hepatic enzymes‡
Constipation‡
Dry mouth‡
Itchiness‡
Hives - Urticaria
Hypersensitivity‡
Muscle spasms‡
Bleeding time prolonged
Aspartate aminotransferase increased
Laboratory test abnormal
Weight loss
Elevated blood creatinine
Cardiac arrest
Arrhythmia
Atrial flutter
Anaemia
Leucopenia
Lymphadenopathy
Coma
Stroke
Transient ischemic attack
Fainting
Myoclonus
Hypoaesthesia
Paraesthesia
Glaucoma
Conjunctivitis
Ear disorder
Pulmonary oedema
Asthma
Shortness of breath
Nose bleed
Cough
Rectal haemhorrhage
Colitis
Gastritis
Indigestion
Difficulty swallowing
Gastrointestinal disorder
Gastrointestinal haemorrhage
Bullous dermatitis
Hair loss
Hirsutism
Hyperhidrosis
Joint pain
Joint stiffness
Back pain
Muscle twitching
Muscle aches
Hyperparathyroidism
Hyperkalaemia
Hypocalcemia
Breast cancer
Sepsis
Pneumonia
Infection
Pharyngitis
Vaginal infection
Influenza
High blood pressure
Hypotension
Gait disturbance
Injection site pain
Fever
Chest pain
Condition aggravated
Muscle weakness
Malaise
Thirst
Breast pain
Impotence
Confusional state
Delirium
Depersonalization
Agitation
Insomnia
Nervousness
‡ These are adverse effects only seen in patients with grade 3 or 4 chronic kidney disease.
† These are adverse effects only seen in patients with grade 5 chronic kidney disease.